 By Netan Choudhry, M.D, FRCSC and Jennifer George
By Netan Choudhry, M.D, FRCSC and Jennifer George
Advances in ophthalmic surgery now make it possible to eliminate or significantly reduce the need to wear glasses or contact lenses, even for those with very large refractive errors requiring thick lenses. Surgery can modify the eye to focus light rays correctly on the retina. Many operations can reduce or correct nearsightedness, farsightedness and astigmatism. Newly developed intraocular lenses can even correct presbyopia – the reduced ability to focus from far to near that people begin to experience in their 40s.
There are two basic types of corrective refractive surgery. One modifies the curvature of the cornea, the outer surface of the eye. The other alters its internal optics by either replacing the natural lens of the eye or using an intraocular lens in addition to the natural lens.
Refractive surgery became popular in the U.S. through radial keratotomy (RK), an operation that was introduced in the early 1980s. In this operation, incisions made in the outer part of the cornea result in the flattening of the central part of the cornea. This can correct mild-to-moderate nearsightedness. Astigmatism can be corrected with astigmatic keratotomy, which involves making circumferential incisions in the outer part of cornea. Radial keratotomy has been largely replaced by better procedures but astigmatic keratotomy is still performed widely, especially together with cataract surgery. With cataract surgery, the pre-existing spherical refractive error can be improved by choosing an intraocular lens of appropriate power. Astigmatism can be corrected by utilizing a toric intraocular lens or by making astigmatic keratotomy incisions at the time of cataract surgery or later. Additionally, an excimer laser can be used before or after cataract surgery to increase the accuracy of the refractive correction.
Femtosecond laser (FSL) technology has been widely used in various refractive surgery applications in recent years. Studies have suggested decreased phacoemulsification energy use with FSL when it is used for cataract surgery, with the potential advantages of more precise corneal incisions and capsulotomy formation. The precision of FSL can allow a surgeon to create the circular opening with the exact intended size, shape and location. Clinical studies indicate that the circular opening is almost 10 times more accurate than the manual alternative.
Using FSL, surgery is highly customizable. Patients will receive more precise treatment and because FSL is less invasive, the procedure results in little to no discomfort. The added lower-energy approach of FSL also results in faster recovery times, placing this new approach on the cutting edge. Femtosecond laser technology has recently been used as a new technique for performing laser vision correction on the cornea. Small incision lenticule extraction, or SMILE, is a new way of performing laser vision correction on the cornea. It uses a femtosecond laser to separate a thin lenticule, or disc-shaped segment of corneal tissue, from within the cornea. This disc is then removed through a very small incision and the resulting change in the corneal shape corrects the patient’s nearsightedness, also known as myopia.
A major advantage of SMILE over LASIK is that it is not dependent on the creation of a flap. LASIK flaps present a host of potential complications, from interface inflammation (diffuse lamellar keratitis, or DLK), striae in the flap, or frank flap dehiscence. As the corneal surface and corneal nerves are less injured with SMILE than LASIK, there is potential for less post-operative ocular surface irritation and dry eye. There is also a theoretical reduction in the risk of post-operative ectasia as well.
For surgeons, a major benefit of SMILE over LASIK is that SMILE only utilizes a femtosecond laser. LASIK utilizes a femtosecond laser to create the flap, and uses an excimer laser to perform the actual ablation. SMILE offers a refractive surgical option with comparable efficacy, predictability and rapidity of visual rehabilitation to LASIK, but with only one laser.
Since the SMILE lenticule is extracted as a single piece, it may be possible to use the lenticule for other purposes. One suggestion is that refracted lenticules might be stored for reimplantation at a later time. This might provide a method of tissue restoration in ectatic corneas and could afford an opportunity for reversing the myopic correction in patients who might progress to presbyopia. Refractive lenticule reimplantation has been demonstrated in rabbits that have been cryopreserved for a month.
The ideal surgical candidate for SMILE is a moderate myope with relatively low astigmatism. It has been used to treat up to 5 diopters of astigmatism and up to 10 diopters of myopia but surgeons often note that low myopic treatments are somewhat more challenging as the lenticule created is relatively thin. Unfortunately, hyperopic treatment with SMILE is still experimental, and there is concern for hyperopic regression after treatment. So, for the hyperopic population, LASIK remains a first-line refractive option.
For a certain subgroup of patients with dry eyes and other corneal surface issues, SMILE may provide a better outcome. For others with irregular corneal shapes, LASIK is still the best option. As with all laser eye surgery, the best procedure depends on a number of factors and should be recommended by a surgeon to provide the optimal result.


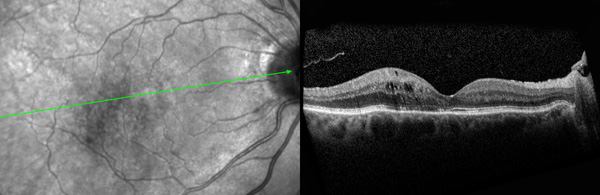
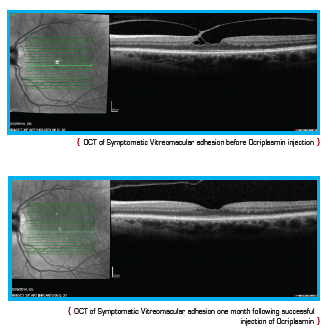 Current treatment options for symptomatic VMA include a period of watchful waiting, surgical removal of the vitreous gel known as vitrectomy, and the intraocular injection of medication. During vitrectomy, an ophthalmologist uses a small, combined cutting/suction tool in order to remove part of the vitreous gel, thus relieving the adhesion between the vitreous and retina. The removed vitreous gel is then replaced with fluid or a temporary gas bubble. Vitrectomy is only performed when patients are at risk of severe visual impairment or loss of central vision.
Current treatment options for symptomatic VMA include a period of watchful waiting, surgical removal of the vitreous gel known as vitrectomy, and the intraocular injection of medication. During vitrectomy, an ophthalmologist uses a small, combined cutting/suction tool in order to remove part of the vitreous gel, thus relieving the adhesion between the vitreous and retina. The removed vitreous gel is then replaced with fluid or a temporary gas bubble. Vitrectomy is only performed when patients are at risk of severe visual impairment or loss of central vision.